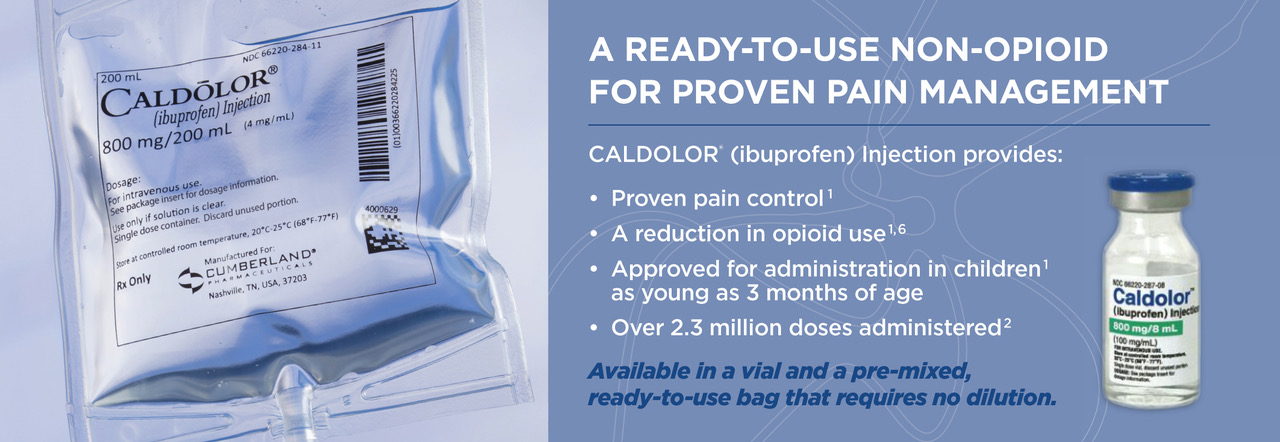
IV Management of Pain and Fever
CALDOLOR is indicated in adults and pediatric patients three months and older for the:
- Management of mild to moderate pain and the management of moderate to severe pain as an adjunct to opioid analgesics
- Reduction of fever
CALDOLOR in vials must be diluted prior to use. Infusion of the drug product in the vial without dilution can cause hemolysis. CALDOLOR in the vial should not be given as an IV bolus or IM injection.1
CALDOLOR is also available in a ready-to-use bag that requires no dilution prior to use.1
WARNING: RISK OF SERIOUS CARDIOVASCULAR AND GASTROINTESTINAL EVENTS1
Cardiovascular Thrombotic Events
- Non-steroidal anti-inflammatory drugs (NSAIDs) cause an increased risk of serious cardiovascular thrombotic events, including myocardial infarction and stroke, which can be fatal. This risk may occur early in treatment and may increase with duration of use.
- CALDOLOR is contraindicated in the setting of coronary artery bypass graft (CABG) surgery.
- NSAIDs cause an increased risk of serious gastrointestinal (GI) adverse events including bleeding, ulceration, and perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Elderly patients and patients with a prior history of peptic ulcer disease and/or GI bleeding are at greater risk for serious GI events.
INDICATIONS AND USAGE1
CALDOLOR is indicated in adults and pediatric patients three months and older for the:
- Management of mild to moderate pain and the management of moderate to severe pain as an adjunct to opioid analgesics
- Reduction of fever
IMPORTANT DOSAGE AND ADMINISTRATION INSTRUCTIONS1
CALDOLOR Injection 800 mg/8 mL (100 mg/mL) vials must be diluted prior to administration.
CALDOLOR Injection 800 mg/200 mL (4 mg/mL) bags are ready to use and require no dilution prior to use.
Do not exceed 3,200 mg total daily dose in adults. Do not exceed 40 mg/kg or 2,400 mg, whichever is less, total daily dose in pediatric patients less than 17 years of age. In pediatric patients 3 months to less than 6 months of age, the dosage is limited to a single dose not to exceed 10 mg/kg or 100 mg, whichever is less.
IMPORTANT SAFETY INFORMATION1
CONTRAINDICATIONS
CALDOLOR is contraindicated in patients with known hypersensitivity (e.g., anaphylactic reactions and serious skin reactions) to ibuprofen or any components of the drug product, and in patients who have a history of asthma, urticaria, or other allergic-type reactions after taking aspirin or other NSAIDs. Severe, sometimes fatal, anaphylactic reactions to NSAIDs have been reported in such patients. CALDOLOR is contraindicated in the setting of coronary artery bypass graft (CABG) surgery.
WARNINGS AND PRECAUTIONS1
CALDOLOR should be used with caution in patients with known cardiovascular (CV) disease or risk factors for CV disease, a history of peptic ulcer disease and/or GI bleeding, liver disease or symptoms of, hypertension, and heart failure. When used in such patients, attention to using the lowest effective dose for the shortest time period is important to reduce the risk of serious adverse events. Avoid use in pregnant women starting at 30 weeks gestation.
ADVERSE REACTIONS1
The most common adverse reactions are nausea, flatulence, vomiting, headache, hemorrhage and dizziness (>5%). The most common adverse reactions in pediatric patients are infusion site pain, vomiting, nausea, anemia and headache (≥2%).